Aβ(1-42) tetramer and octamer structures reveal edge conductivity

Carulla Laβ (@carullalab) / X

Molecular dynamics simulations reveal the importance of amyloid-beta oligomer β-sheet edge conformations in membrane permeabilization - ScienceDirect

Single-molecule Mapping of Amyloid-β Oligomer Insertion into Lipid
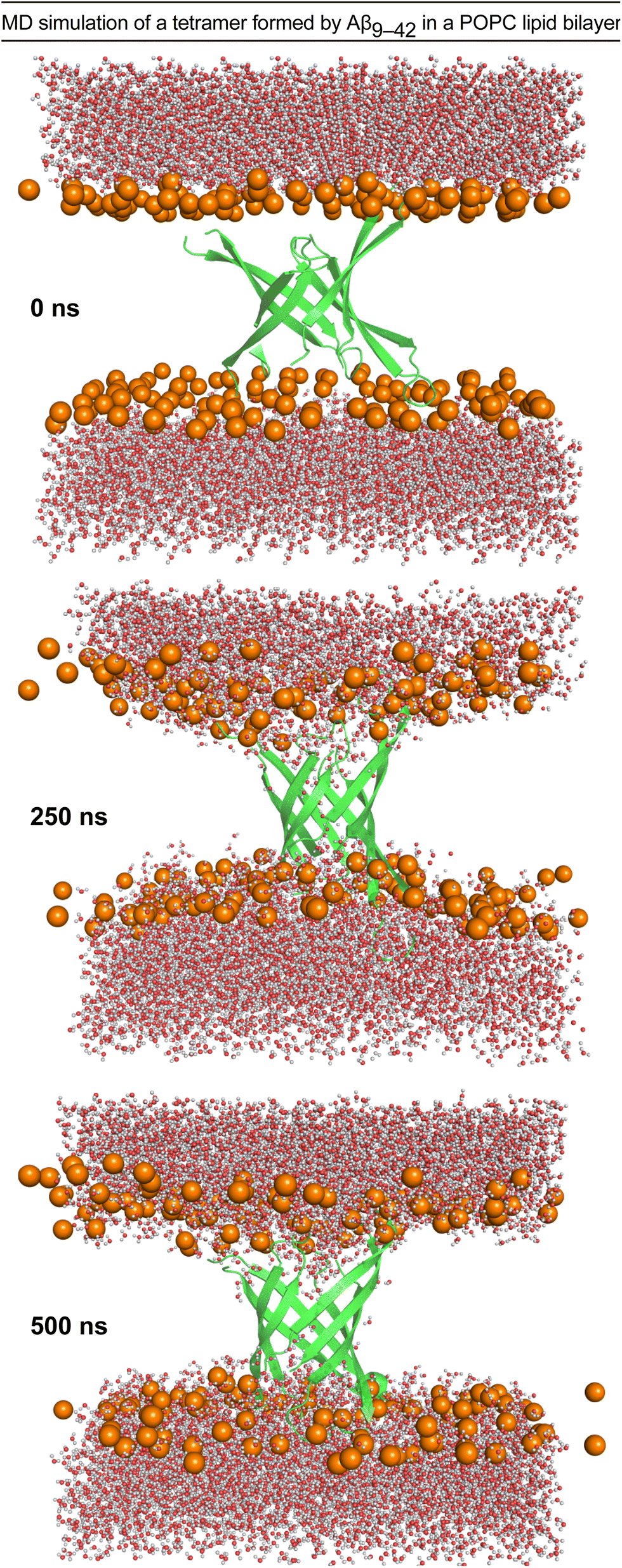
A β-barrel-like tetramer formed by a β-hairpin derived from Aβ

PDF) Aβ(1-42) tetramer and octamer structures reveal edge pores as a mechanism for membrane damage

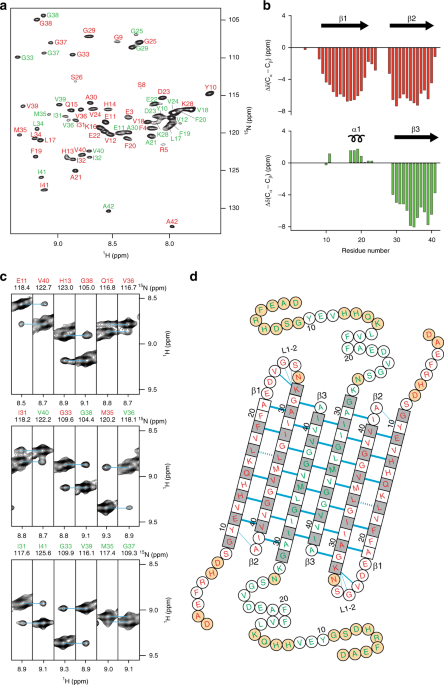
3D structure of the Aβ(1-42) tetramer prepared in DPC a Ribbon diagram

IJMS, Free Full-Text

Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage
Why are the root causes of amyloid-associated diseases so misunderstood and treatments so inadequate?

RCSB PDB - 6RHY: Structure of pore-forming amyloid-beta tetramers
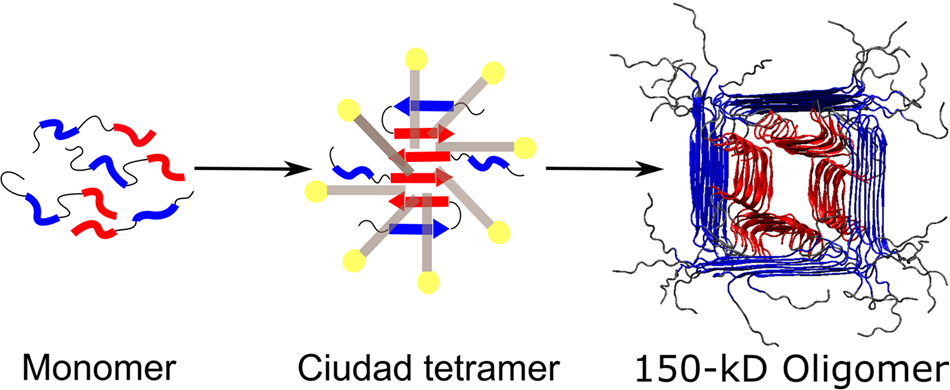
A common pathway for detergent-assisted oligomerization of Aβ42
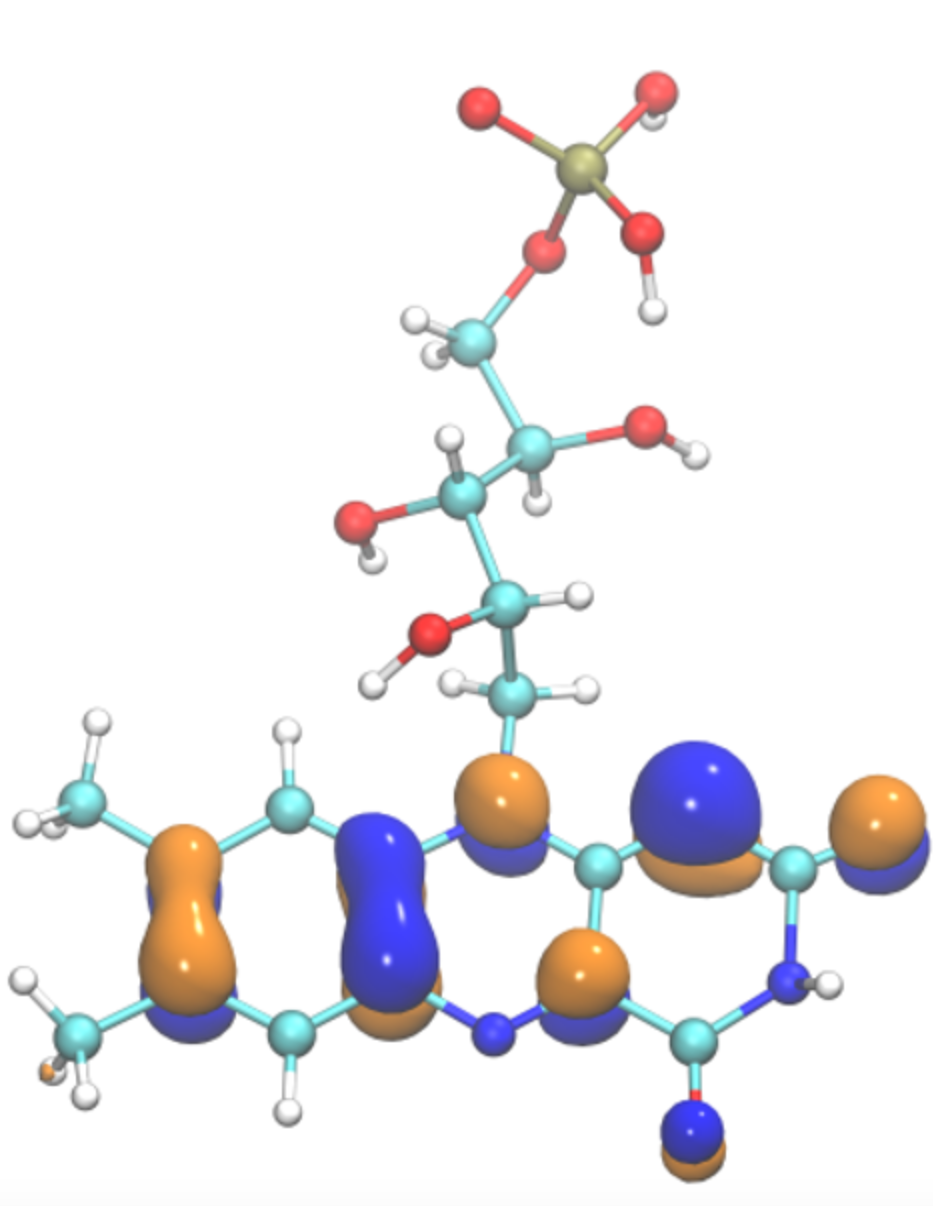
Computational Structural Biology and Molecular Biophysics









