Hemoglobin & Myoglobin: 4. Dissociation Curves - Biochemistry Flashcards

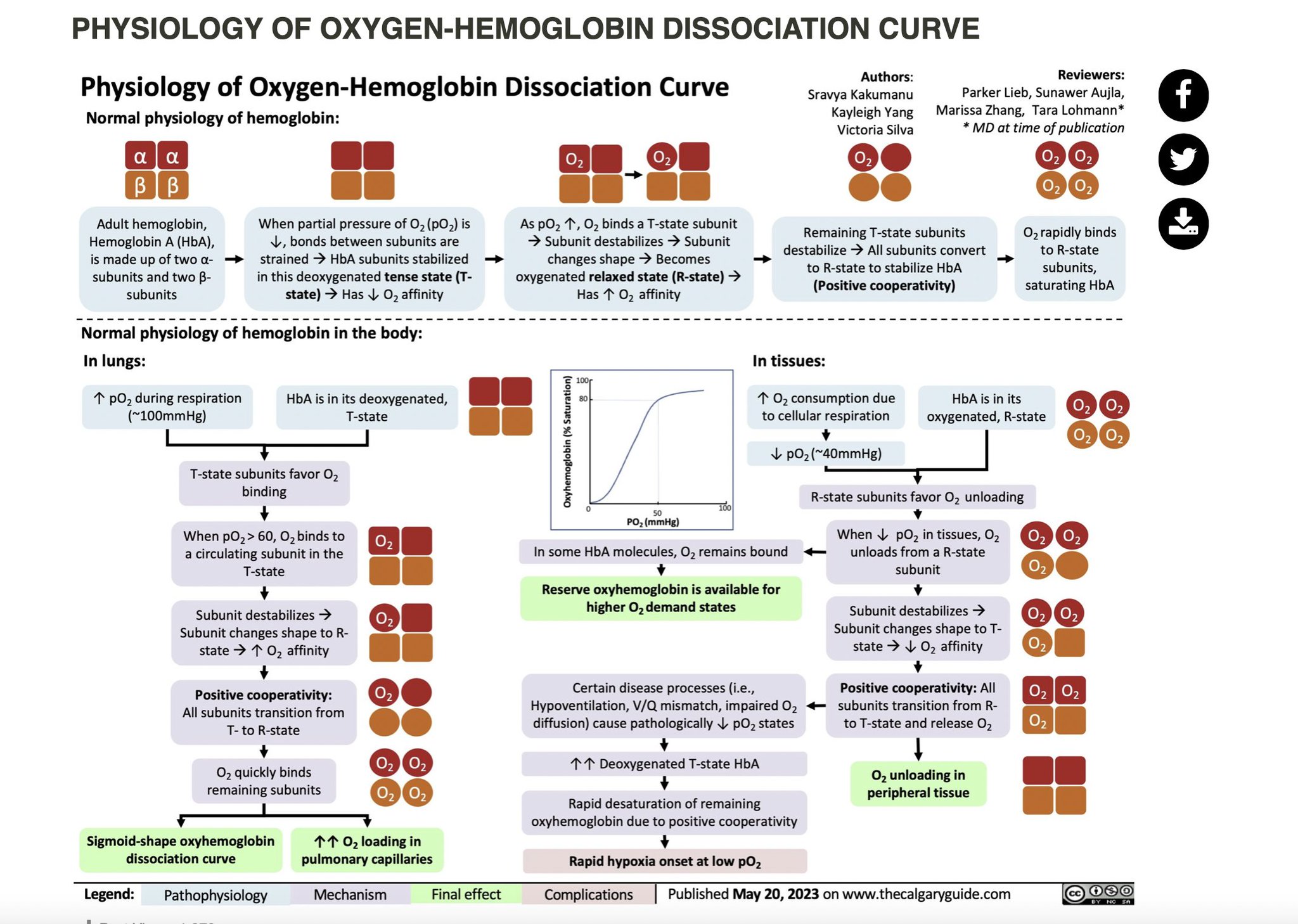
Dissociation Curves We can compare compare the binding properties of both myoglobin and hemoglobin by drawing their dissociation curves. These curves measure their relative affinities for oxygen. • We draw a graph and label the x-axis oxygen partia
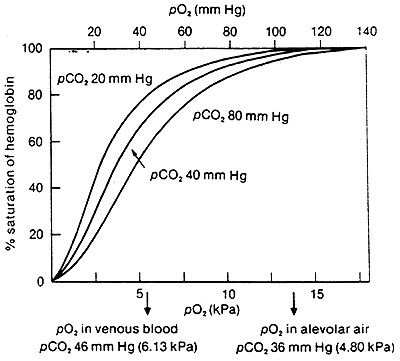
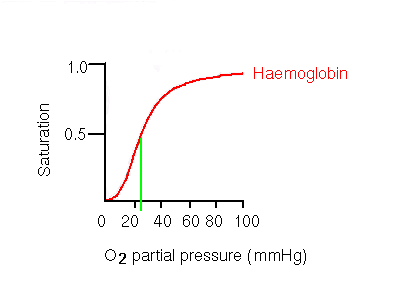
Dissociation Curves We can compare compare the binding properties of both myoglobin and hemoglobin by drawing their dissociation curves. These curves measure their relative affinities for oxygen. • We draw a graph and label the x-axis oxygen partial pressure (torr). Number it 0 to 120. – 30 torr is ~ the partial pressure of oxygen in the body's tissues. – 100 torr is ~ the partial pressure in the lungs. • The y-axis % oxygen saturation: it's numbered 0 to 100. We will use it to compare the oxygen binding patterns of both hemoglobin and myoglobin. oxygen binding curve for hemoglobin and myoglobin Hemoglobin • We draw a sigmoidal curve that plateaus just below 100% saturation. • Cooperative binding produces this sigmoidal shape. – As one oxygen molecule binds, hemoglobin's affinity for additional oxygen increases, and its percent saturation rapidly increases. • We show that hemoglobin reaches half saturation in the peripheral tissues – it responds to oxygen availability and releases it when partial pressure is low. Myoglobin • We draw a hyperbolic curve to the left of the hemoglobin curve, a much simpler binding pattern that corresponds to myoglobin's single heme group. – Myoglobin has a high affinity for oxygen, and does not release it until the partial pressure is very low. – These binding properties correspond to myoglobin's role in oxygen storage. – We label the early portion of the curve "Exercising muscle" and the plateau "Muscle at rest." Myoglobin vs. Hemoglobin • Myoglobin releases oxygen in response to the muscle's immediate needs. • Hemoglobin's cooperative binding allows it to respond to changes in oxygen availability. Fetal hemoglobin dissociation curve • As a clinical correlation, we show that the fetal hemoglobin dissociation curve is to the left of the adult hemoglobin curve. • This is because it has a greater affinity for oxygen to facilitate oxygen transfer from the maternal hemoglobin to the fetus; fetal oxygen supplies come from the mother.
Which gas combines with haemoglobin to damage its oxygen carrying property? - Quora
What is the O2-Hemoglobin dissociation curve, and what factors and how can affect it? - Quora

Exam 3 Material Flashcards
Myoglobin/Hemoglobin
Protein Interactions: Haemoglobin
What are the functions of hemoglobin and myoglobin in blood? - Quora

Biochem Midtern 2 Flashcards

AK Lectures - Oxygen Binding Curve for Myoglobin and Hemoglobin
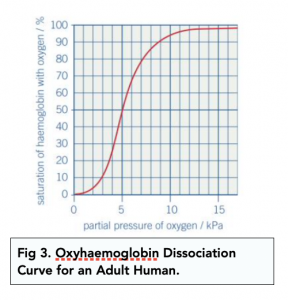
Transport of Oxygen (A-level Biology) - Study Mind
Structural Biochemistry/Hemoglobin - Wikibooks, open books for an open world

BioChem: L3P2 - Proteins Flashcards
Solved] Which of the following statements about myoglobin and hemoglobin is
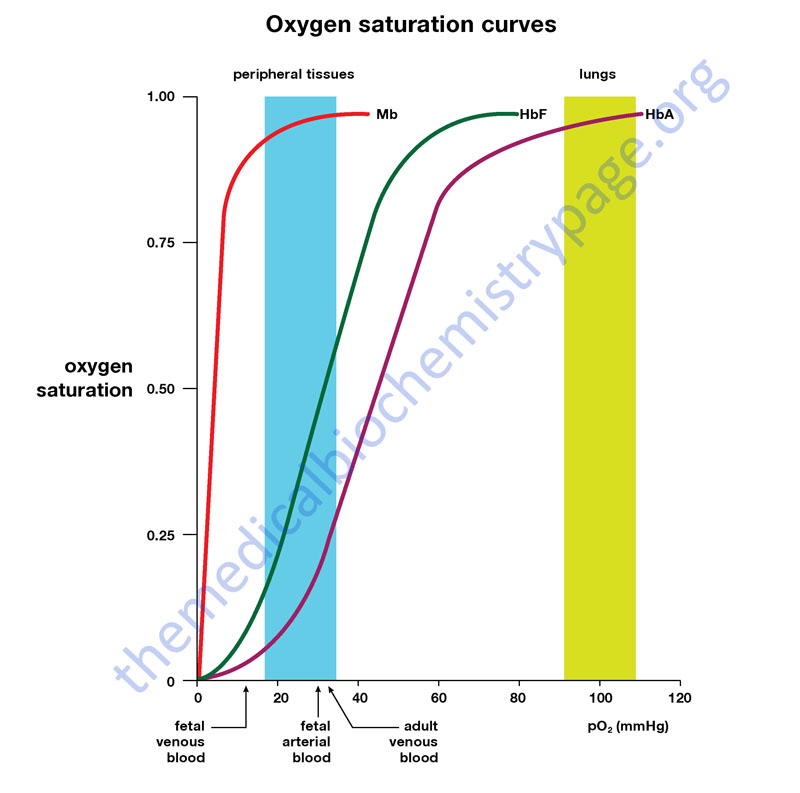
Hemoglobin and Myoglobin - The Medical Biochemistry Page