Which of the following statements is/are correct? (a) all real gases are less compressible

Which of the following statements is/are correct? (a) all real gases are less compressible than ideal gas at high pressures? (6) hydrogen and helium are more co

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

For A Real Gas At 25∘C Temperature And High Pressure (99, 59% OFF

PPT - 6 Gases PowerPoint Presentation, free download - ID:352441
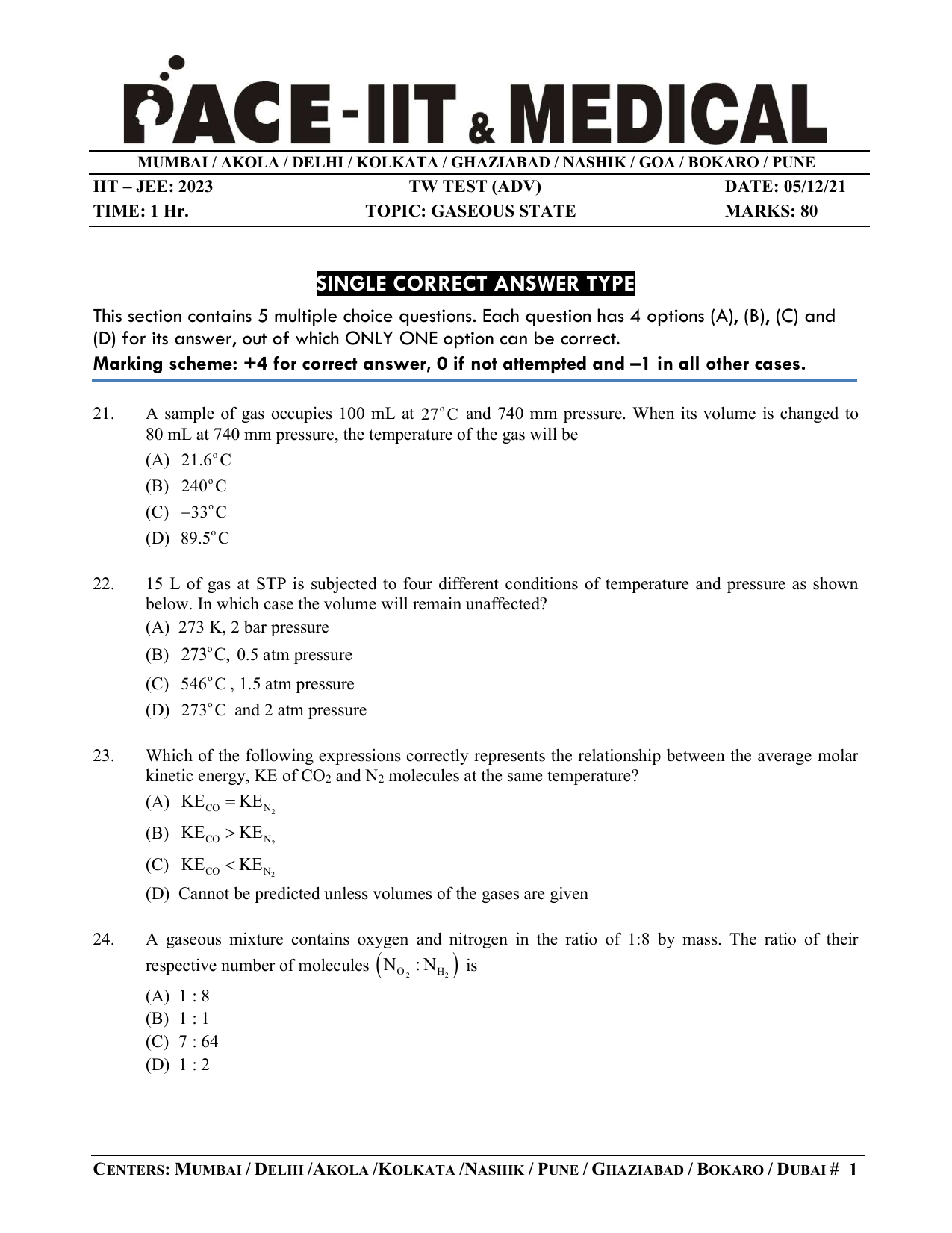
Gaseous State (ADV) Question Paper

Which of the following statements is/are correct? (a) all real gases are less compressible
Why there is different between the value of compressibility factor at critical point between real and ideal gas? - Quora

What Exactly is The Compressibility of Fluids?
How can a gas be ideal at a high pressure and low temperature? - Quora

SOLVED: Make judgment of the following statements and justify your

Compressibility: Definition, How It Works, Calculation, and
Equations of Compressible and Incompressible Flow in Fluid Dynamics, System Analysis Blog

Non-Ideal Gas Behavior Chemistry: Atoms First
What is adiabatic index? Why is its value always greater than unity? - Quora

Which of the following statements is/are correct? (a) all real gases are less compressible
Ideal fluids, bernouilli's law



)